Jaeger’s method to determine surface tension of liquid pdf
2 Yao-Yuan Chang, Shi-Yow Lin, Surface tension measurement of glass melts using sessile or pendant drop methods, Journal of the Taiwan Institute of Chemical Engineers, 2011, 42, 6, 922CrossRef 3 C. A. Smolders , E. M. Duyvis , Contact angles; wetting and de-wetting of mercury: Part I.
DROP WEIGHT METHOD Drop formation of liquid by capillary tube is step which depends upon the surface tension of that liquid. The size of falling off from end of capillary tube depends upon the S.T. fo the liquid and the size
This Test Guideline describes methods to determine the surface tension (in N/m) of aqueous solutions. The methods are based on the measurement of the force which it is necessary to exert vertically on a stirrup or ring, in contact with the surface of the liquid, in order to separate it from the surface, or on a plate, with an edge in contact
jaeger’s method to determine surface tension of liquid measurement of surface tension by capillary rise method describe in details jaeger method to determine surface tension of liquid surface tension of water by capillary rise method using travelling microscope surface tension by jaeger’s method experiment pdf surface tension by capillary rise method experiment There are several …
The expression which relates the liquid surface tension with the pressure difference is obtained by performing an analysis of the work done by the air pressure to increase the volume and the surface area of the bubble while it expands into the sample liquid Equation 1.
The surface tension of molten glass is of prime importance in practically all operations of manufacturing glass wares, beginning with the fining process, and continuing until the glass becomes rigid.
Surface Tension as a Function of Temperature and Concentration of Liquids. U. P. SHINDE1, S. S. CHOUGULE1, follows 1) Capillary rise method 2) Jaeger’s method 3) Ferguson and Kennedy’s method 4) Quincke’s method etc. From the above methods the capillary rise method is simple, easy and less expensive.
tension at a liquid-air surface may be obtained by measuring the period of oscilla- tion of a small drop of the liquid, but the method is hardly likely to commend itself for widespread use, especially in technological laboratories.
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
As the surface tension of the liquid jet changes because of surfac- taut diffusion to the air/liquid interface, each succeeding wave will have longer wavelength; i.e., will have lower surface tension …
Determination of the surface tension of liquid stainless
https://youtube.com/watch?v=DxJXkItB8AM
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
A static method of measuring the surface tension of a liquid is presented. Jaeger’s method is modified by replacing the pressure source with a variable pressure head. By using this method, stationary air bubbles are obtained thus resulting in controllable external parameters. (Author/KR
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets
Determine the surface tension of olive oil as a function of temperature. 2. Determine the surface tension of water/methanol mixtures as functions of the mixture ratio. Set-up and procedure Perform the experimental set-up according to Fig. 1. The measuring ring is carefully degreased with alcohol, rinsed In distilled water and dried. The ring is attached to the left arm of the torsion
The surface tension γ is the magnitude F of the force exerted parallel to the surface of a liquid divided by the length L of the line over which the force acts: γ=
The procedure to determine the surface tension of a given liquid is as follows: Measure the ambient temperature and fill the burette with the liquid to be analyzed. The drop formation time is regulated slowly enough to avoid hydrodynamics effects.
An optical measuring method has been applied to determine the dynamic surface tension of aqueous solutions of heptanol. The method uses the frequency of an oscillating liquid droplet as an indicator of the surface tension of the liquid. Droplets with diameters in the range between 100 and 200 μm
surface properties of fused salts and glasses: i sessile-drop method for determining surface tension and density of viscous liquids at high temperatures *
In physics, the maximum bubble pressure method, or in short bubble pressure method, is a technique to measure the surface tension of a liquid, with surfactants.
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid

The temperature coefficient of the surface tension (dσ/dT) of liquid stainless steel was found to change from negative to positive at a sulphur concentration of about 30 mass ppm in the steel. Nitrogen was found to have little effect on the surface tension of liquid stainless steel.
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter (N m −1 ), is high and shows a slight increase as the temperature falls from 100 (0.0589 N m −1 ) to 0 °C (0.0765 N m −1 ).
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
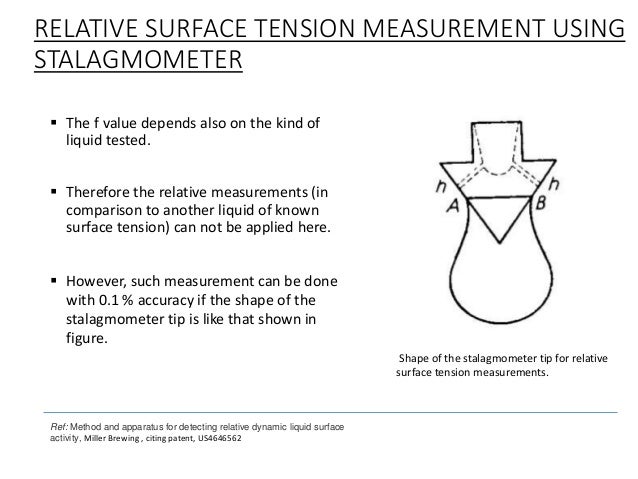
surface tension of the liquid. This phenomenon is used to determine the surface tension using stalagmometric method (method of counting drops). Stalagmometer (Fig. 1a) is a device made up of a glass bulb with marked above and below the bulb indicators designating specific volume of liquid, ended capillary. To measure the surface tension of the test liquid fill of stalagmometer and allow it to
Critical Assessment of the Surface Tension determined by
Show transcribed image text A method for determining the surface tension of a liquid is to find the force needed to pull a wire ring from the surface of a liquid. For this purpose, usually platinum rings are used, since they form zero contact angles with the outermost and innermost peripheries of the wire. Determine the force required to pull such a ring of diameter 0.4 m from water above the
To determine the surface tension of water by capillary rise method. APPARATUS Capillary tube and a tipped pointer clamped in a stand, travelling microscope, clean water in a beaker. THEORY Surface tension, T = r h pg ∕2cosϴ PROCEDURE Measurement of capillary rise 1. Find the least count of the travelling microscope for the horizontal and the verticalscale. Record the same in the note-book
By doing measurements with m number of liquids on the same surface, we can calculate m different components of the surface energy, if the corresponding components of the liquids are known.
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets that can fit onto a penny without spilling over for each liquid. The liquid that can fit the most droplets onto the penny has the highest surface tension, because it can hold onto itself the tightest
Just a lab report. I. Introduction: At a given temperature and pressure, a body of liquid does work against the cohesive forces in the interior in order for the molecules to make a transfer into the surface and increase the surface area.
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
Full text of “Measurement of Surface Tension” JAEGER’S METHOD When the column of liquid that rise& in a tube dipping vertically into a liquid is slowly forced down by applied gas pressure, it is observed that the pressure steadily increases to a well-marked maxi- mum just before a bubble escapes from the end of the tube. The value of this maximum depends upon the surface tension and the
The Surface tensions of molten glass part I surface
This will allow us to determine the Surface Energy (ES) of this liquid. Also, the Surface Tension of aqueous solutions of Ethanol and n-Butanol will be measured as a function of solute concentration. Using this data, we will demonstrate that for a sufficiently dilute solution, n-Butanol, a Surface Active molecule, behaves as a Two Dimensional Ideal Gas. This data will also allow us to
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
surface.Itmayhaveanyvaluebetweenand180°,eachincluded. Ifthe wallhasan absolutely sharpedge atthelineofcontact,the concept ofa tangent tothe wall at and acrossthe line ofcontact
The third method also allows the calculation of the surface tension between a solid spherical nanoparticle and a liquid, which makes a direct link to the motivating example given above.
Liquid-Liquid Interfacial Tension Measurements October 18, 2006 This note discusses liquid-liquid interfacial tension measurements on an oil-water system using an FTA200. In particular, it shows how to recognize certain difficulties in these measurements. A #20 “J” needle (0.914mm OD) introduced oil, the l ighter phase, as a rising bubble into the surrounding water phase. Good IFT
To determine the surface tension of a liquid (water) by Jaeger’s method. Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer
Surface tension of vitreous enamel frits at and near

Methods SINTERFACE
Solved A Method For Determining The Surface Tension Of A
A mechanics bsc exp 020 to determine the surface tension
Conducting Surface Tension Measurements For Compliance


AIM DETERMINATION OF SURFACE TENSION OF LIQUID
Measurement of dynamic surface tension by the oscillating
Liquid-Liquid Interfacial Tension Measurements

Instruction for the Laboratory of Biophysics
https://youtube.com/watch?v=7LSyPofVZlg
Surface Tension Lab Report Surface Tension Solution
OECD iLibrary Test No. 115 Surface Tension of Aqueous
AIM DETERMINATION OF SURFACE TENSION OF LIQUID
The surface tension of molten glass is of prime importance in practically all operations of manufacturing glass wares, beginning with the fining process, and continuing until the glass becomes rigid.
This Test Guideline describes methods to determine the surface tension (in N/m) of aqueous solutions. The methods are based on the measurement of the force which it is necessary to exert vertically on a stirrup or ring, in contact with the surface of the liquid, in order to separate it from the surface, or on a plate, with an edge in contact
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
surface properties of fused salts and glasses: i sessile-drop method for determining surface tension and density of viscous liquids at high temperatures *
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
The temperature coefficient of the surface tension (dσ/dT) of liquid stainless steel was found to change from negative to positive at a sulphur concentration of about 30 mass ppm in the steel. Nitrogen was found to have little effect on the surface tension of liquid stainless steel.
Instruction for the Laboratory of Biophysics
Surface tension of vitreous enamel frits at and near
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
The third method also allows the calculation of the surface tension between a solid spherical nanoparticle and a liquid, which makes a direct link to the motivating example given above.
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter (N m −1 ), is high and shows a slight increase as the temperature falls from 100 (0.0589 N m −1 ) to 0 °C (0.0765 N m −1 ).
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
Surface tension of vitreous enamel frits at and near
The Surface tensions of molten glass part I surface
surface tension of the liquid. This phenomenon is used to determine the surface tension using stalagmometric method (method of counting drops). Stalagmometer (Fig. 1a) is a device made up of a glass bulb with marked above and below the bulb indicators designating specific volume of liquid, ended capillary. To measure the surface tension of the test liquid fill of stalagmometer and allow it to
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
jaeger’s method to determine surface tension of liquid measurement of surface tension by capillary rise method describe in details jaeger method to determine surface tension of liquid surface tension of water by capillary rise method using travelling microscope surface tension by jaeger’s method experiment pdf surface tension by capillary rise method experiment There are several …
The procedure to determine the surface tension of a given liquid is as follows: Measure the ambient temperature and fill the burette with the liquid to be analyzed. The drop formation time is regulated slowly enough to avoid hydrodynamics effects.
Show transcribed image text A method for determining the surface tension of a liquid is to find the force needed to pull a wire ring from the surface of a liquid. For this purpose, usually platinum rings are used, since they form zero contact angles with the outermost and innermost peripheries of the wire. Determine the force required to pull such a ring of diameter 0.4 m from water above the
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets that can fit onto a penny without spilling over for each liquid. The liquid that can fit the most droplets onto the penny has the highest surface tension, because it can hold onto itself the tightest
An optical measuring method has been applied to determine the dynamic surface tension of aqueous solutions of heptanol. The method uses the frequency of an oscillating liquid droplet as an indicator of the surface tension of the liquid. Droplets with diameters in the range between 100 and 200 μm
This Test Guideline describes methods to determine the surface tension (in N/m) of aqueous solutions. The methods are based on the measurement of the force which it is necessary to exert vertically on a stirrup or ring, in contact with the surface of the liquid, in order to separate it from the surface, or on a plate, with an edge in contact
Methods SINTERFACE
Liquid-Liquid Interfacial Tension Measurements
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets that can fit onto a penny without spilling over for each liquid. The liquid that can fit the most droplets onto the penny has the highest surface tension, because it can hold onto itself the tightest
This will allow us to determine the Surface Energy (ES) of this liquid. Also, the Surface Tension of aqueous solutions of Ethanol and n-Butanol will be measured as a function of solute concentration. Using this data, we will demonstrate that for a sufficiently dilute solution, n-Butanol, a Surface Active molecule, behaves as a Two Dimensional Ideal Gas. This data will also allow us to
By doing measurements with m number of liquids on the same surface, we can calculate m different components of the surface energy, if the corresponding components of the liquids are known.
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
The surface tension of molten glass is of prime importance in practically all operations of manufacturing glass wares, beginning with the fining process, and continuing until the glass becomes rigid.
To determine the surface tension of water by capillary rise method. APPARATUS Capillary tube and a tipped pointer clamped in a stand, travelling microscope, clean water in a beaker. THEORY Surface tension, T = r h pg ∕2cosϴ PROCEDURE Measurement of capillary rise 1. Find the least count of the travelling microscope for the horizontal and the verticalscale. Record the same in the note-book
The temperature coefficient of the surface tension (dσ/dT) of liquid stainless steel was found to change from negative to positive at a sulphur concentration of about 30 mass ppm in the steel. Nitrogen was found to have little effect on the surface tension of liquid stainless steel.
surface properties of fused salts and glasses: i sessile-drop method for determining surface tension and density of viscous liquids at high temperatures *
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets
Just a lab report. I. Introduction: At a given temperature and pressure, a body of liquid does work against the cohesive forces in the interior in order for the molecules to make a transfer into the surface and increase the surface area.
DROP WEIGHT METHOD Drop formation of liquid by capillary tube is step which depends upon the surface tension of that liquid. The size of falling off from end of capillary tube depends upon the S.T. fo the liquid and the size
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
tension at a liquid-air surface may be obtained by measuring the period of oscilla- tion of a small drop of the liquid, but the method is hardly likely to commend itself for widespread use, especially in technological laboratories.
Maximum bubble pressure method Wikipedia
Critical Assessment of the Surface Tension determined by
jaeger’s method to determine surface tension of liquid measurement of surface tension by capillary rise method describe in details jaeger method to determine surface tension of liquid surface tension of water by capillary rise method using travelling microscope surface tension by jaeger’s method experiment pdf surface tension by capillary rise method experiment There are several …
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter (N m −1 ), is high and shows a slight increase as the temperature falls from 100 (0.0589 N m −1 ) to 0 °C (0.0765 N m −1 ).
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
To determine the surface tension of a liquid (water) by Jaeger’s method. Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer
Maximum bubble pressure method Wikipedia
Liquid-Liquid Interfacial Tension Measurements
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
This Test Guideline describes methods to determine the surface tension (in N/m) of aqueous solutions. The methods are based on the measurement of the force which it is necessary to exert vertically on a stirrup or ring, in contact with the surface of the liquid, in order to separate it from the surface, or on a plate, with an edge in contact
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
As the surface tension of the liquid jet changes because of surfac- taut diffusion to the air/liquid interface, each succeeding wave will have longer wavelength; i.e., will have lower surface tension …
DROP WEIGHT METHOD Drop formation of liquid by capillary tube is step which depends upon the surface tension of that liquid. The size of falling off from end of capillary tube depends upon the S.T. fo the liquid and the size
jaeger’s method to determine surface tension of liquid measurement of surface tension by capillary rise method describe in details jaeger method to determine surface tension of liquid surface tension of water by capillary rise method using travelling microscope surface tension by jaeger’s method experiment pdf surface tension by capillary rise method experiment There are several …
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter (N m −1 ), is high and shows a slight increase as the temperature falls from 100 (0.0589 N m −1 ) to 0 °C (0.0765 N m −1 ).
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
surface tension of the liquid. This phenomenon is used to determine the surface tension using stalagmometric method (method of counting drops). Stalagmometer (Fig. 1a) is a device made up of a glass bulb with marked above and below the bulb indicators designating specific volume of liquid, ended capillary. To measure the surface tension of the test liquid fill of stalagmometer and allow it to
Just a lab report. I. Introduction: At a given temperature and pressure, a body of liquid does work against the cohesive forces in the interior in order for the molecules to make a transfer into the surface and increase the surface area.
This will allow us to determine the Surface Energy (ES) of this liquid. Also, the Surface Tension of aqueous solutions of Ethanol and n-Butanol will be measured as a function of solute concentration. Using this data, we will demonstrate that for a sufficiently dilute solution, n-Butanol, a Surface Active molecule, behaves as a Two Dimensional Ideal Gas. This data will also allow us to
To determine the surface tension of a liquid (water) by Jaeger’s method. Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer
Surface Tension Lab Report Surface Tension Solution
Solved A Method For Determining The Surface Tension Of A
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
Just a lab report. I. Introduction: At a given temperature and pressure, a body of liquid does work against the cohesive forces in the interior in order for the molecules to make a transfer into the surface and increase the surface area.
This will allow us to determine the Surface Energy (ES) of this liquid. Also, the Surface Tension of aqueous solutions of Ethanol and n-Butanol will be measured as a function of solute concentration. Using this data, we will demonstrate that for a sufficiently dilute solution, n-Butanol, a Surface Active molecule, behaves as a Two Dimensional Ideal Gas. This data will also allow us to
A static method of measuring the surface tension of a liquid is presented. Jaeger’s method is modified by replacing the pressure source with a variable pressure head. By using this method, stationary air bubbles are obtained thus resulting in controllable external parameters. (Author/KR
To determine the surface tension of a liquid (water) by Jaeger’s method. Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
OECD iLibrary Test No. 115 Surface Tension of Aqueous
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets that can fit onto a penny without spilling over for each liquid. The liquid that can fit the most droplets onto the penny has the highest surface tension, because it can hold onto itself the tightest
By doing measurements with m number of liquids on the same surface, we can calculate m different components of the surface energy, if the corresponding components of the liquids are known.
The procedure to determine the surface tension of a given liquid is as follows: Measure the ambient temperature and fill the burette with the liquid to be analyzed. The drop formation time is regulated slowly enough to avoid hydrodynamics effects.
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
The third method also allows the calculation of the surface tension between a solid spherical nanoparticle and a liquid, which makes a direct link to the motivating example given above.
A static method of measuring the surface tension of a liquid is presented. Jaeger’s method is modified by replacing the pressure source with a variable pressure head. By using this method, stationary air bubbles are obtained thus resulting in controllable external parameters. (Author/KR
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
To determine the surface tension of water by capillary rise method. APPARATUS Capillary tube and a tipped pointer clamped in a stand, travelling microscope, clean water in a beaker. THEORY Surface tension, T = r h pg ∕2cosϴ PROCEDURE Measurement of capillary rise 1. Find the least count of the travelling microscope for the horizontal and the verticalscale. Record the same in the note-book
To determine the surface tension of a liquid (water) by Jaeger’s method. Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer Jaeger’s apparatus , travelling microscope, scale , beaker, capillary Jets and thermometer
Full text of “Measurement of Surface Tension” JAEGER’S METHOD When the column of liquid that rise& in a tube dipping vertically into a liquid is slowly forced down by applied gas pressure, it is observed that the pressure steadily increases to a well-marked maxi- mum just before a bubble escapes from the end of the tube. The value of this maximum depends upon the surface tension and the
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
Solved A Method For Determining The Surface Tension Of A
jaeger’s method to determine surface tension of liquid measurement of surface tension by capillary rise method describe in details jaeger method to determine surface tension of liquid surface tension of water by capillary rise method using travelling microscope surface tension by jaeger’s method experiment pdf surface tension by capillary rise method experiment There are several …
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
surface.Itmayhaveanyvaluebetweenand180°,eachincluded. Ifthe wallhasan absolutely sharpedge atthelineofcontact,the concept ofa tangent tothe wall at and acrossthe line ofcontact
The surface tension γ is the magnitude F of the force exerted parallel to the surface of a liquid divided by the length L of the line over which the force acts: γ=
The procedure to determine the surface tension of a given liquid is as follows: Measure the ambient temperature and fill the burette with the liquid to be analyzed. The drop formation time is regulated slowly enough to avoid hydrodynamics effects.
tension at a liquid-air surface may be obtained by measuring the period of oscilla- tion of a small drop of the liquid, but the method is hardly likely to commend itself for widespread use, especially in technological laboratories.
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter (N m −1 ), is high and shows a slight increase as the temperature falls from 100 (0.0589 N m −1 ) to 0 °C (0.0765 N m −1 ).
To determine the surface tension of water by capillary rise method. APPARATUS Capillary tube and a tipped pointer clamped in a stand, travelling microscope, clean water in a beaker. THEORY Surface tension, T = r h pg ∕2cosϴ PROCEDURE Measurement of capillary rise 1. Find the least count of the travelling microscope for the horizontal and the verticalscale. Record the same in the note-book
An optical measuring method has been applied to determine the dynamic surface tension of aqueous solutions of heptanol. The method uses the frequency of an oscillating liquid droplet as an indicator of the surface tension of the liquid. Droplets with diameters in the range between 100 and 200 μm
2 Yao-Yuan Chang, Shi-Yow Lin, Surface tension measurement of glass melts using sessile or pendant drop methods, Journal of the Taiwan Institute of Chemical Engineers, 2011, 42, 6, 922CrossRef 3 C. A. Smolders , E. M. Duyvis , Contact angles; wetting and de-wetting of mercury: Part I.
DROP WEIGHT METHOD Drop formation of liquid by capillary tube is step which depends upon the surface tension of that liquid. The size of falling off from end of capillary tube depends upon the S.T. fo the liquid and the size
Instruction for the Laboratory of Biophysics
Critical Assessment of the Surface Tension determined by
tension at a liquid-air surface may be obtained by measuring the period of oscilla- tion of a small drop of the liquid, but the method is hardly likely to commend itself for widespread use, especially in technological laboratories.
DROP WEIGHT METHOD Drop formation of liquid by capillary tube is step which depends upon the surface tension of that liquid. The size of falling off from end of capillary tube depends upon the S.T. fo the liquid and the size
As the surface tension of the liquid jet changes because of surfac- taut diffusion to the air/liquid interface, each succeeding wave will have longer wavelength; i.e., will have lower surface tension …
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
surface properties of fused salts and glasses: i sessile-drop method for determining surface tension and density of viscous liquids at high temperatures *
Full text of “Measurement of Surface Tension” JAEGER’S METHOD When the column of liquid that rise& in a tube dipping vertically into a liquid is slowly forced down by applied gas pressure, it is observed that the pressure steadily increases to a well-marked maxi- mum just before a bubble escapes from the end of the tube. The value of this maximum depends upon the surface tension and the
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
Just a lab report. I. Introduction: At a given temperature and pressure, a body of liquid does work against the cohesive forces in the interior in order for the molecules to make a transfer into the surface and increase the surface area.
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
By doing measurements with m number of liquids on the same surface, we can calculate m different components of the surface energy, if the corresponding components of the liquids are known.
Surface Tension as a Function of Temperature and Concentration of Liquids. U. P. SHINDE1, S. S. CHOUGULE1, follows 1) Capillary rise method 2) Jaeger’s method 3) Ferguson and Kennedy’s method 4) Quincke’s method etc. From the above methods the capillary rise method is simple, easy and less expensive.
Conducting Surface Tension Measurements For Compliance
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
The procedure to determine the surface tension of a given liquid is as follows: Measure the ambient temperature and fill the burette with the liquid to be analyzed. The drop formation time is regulated slowly enough to avoid hydrodynamics effects.
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
As the surface tension of the liquid jet changes because of surfac- taut diffusion to the air/liquid interface, each succeeding wave will have longer wavelength; i.e., will have lower surface tension …
Full text of “Measurement of Surface Tension” JAEGER’S METHOD When the column of liquid that rise& in a tube dipping vertically into a liquid is slowly forced down by applied gas pressure, it is observed that the pressure steadily increases to a well-marked maxi- mum just before a bubble escapes from the end of the tube. The value of this maximum depends upon the surface tension and the
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets
Surface Tension as a Function of Temperature and Concentration of Liquids. U. P. SHINDE1, S. S. CHOUGULE1, follows 1) Capillary rise method 2) Jaeger’s method 3) Ferguson and Kennedy’s method 4) Quincke’s method etc. From the above methods the capillary rise method is simple, easy and less expensive.
An optical measuring method has been applied to determine the dynamic surface tension of aqueous solutions of heptanol. The method uses the frequency of an oscillating liquid droplet as an indicator of the surface tension of the liquid. Droplets with diameters in the range between 100 and 200 μm
In this portion of the lab you will determine which liquid has the highest surface tension: water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of droplets that can fit onto a penny without spilling over for each liquid. The liquid that can fit the most droplets onto the penny has the highest surface tension, because it can hold onto itself the tightest
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
surface.Itmayhaveanyvaluebetweenand180°,eachincluded. Ifthe wallhasan absolutely sharpedge atthelineofcontact,the concept ofa tangent tothe wall at and acrossthe line ofcontact
surface properties of fused salts and glasses: i sessile-drop method for determining surface tension and density of viscous liquids at high temperatures *
Maximum bubble pressure method Wikipedia
Surface Tension Prediction for Liquid Mixtures
Surface tension is the property of a liquid in contact with air or vapor that makes it behave as if it were covered with a thin membrane under tension. For example, if
2 Yao-Yuan Chang, Shi-Yow Lin, Surface tension measurement of glass melts using sessile or pendant drop methods, Journal of the Taiwan Institute of Chemical Engineers, 2011, 42, 6, 922CrossRef 3 C. A. Smolders , E. M. Duyvis , Contact angles; wetting and de-wetting of mercury: Part I.
The surface tension γ is the magnitude F of the force exerted parallel to the surface of a liquid divided by the length L of the line over which the force acts: γ=
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
Just a lab report. I. Introduction: At a given temperature and pressure, a body of liquid does work against the cohesive forces in the interior in order for the molecules to make a transfer into the surface and increase the surface area.
The third method also allows the calculation of the surface tension between a solid spherical nanoparticle and a liquid, which makes a direct link to the motivating example given above.
determine displacements of the bottom of the cylinder from the plane of the undisturbed liquid surface. The test liquid is contained in the J Figures in brackets indicate the literature references at the end of this paper. Journal of Research of the National Bureau of Standards Research Paper 1133 F FIGURE I.- SUljace tension apparatus with j1tmaCe displaced to left to show essential parts. A
Maximum bubble pressure method Wikipedia
Measurement of dynamic surface tension by the oscillating
Show transcribed image text A method for determining the surface tension of a liquid is to find the force needed to pull a wire ring from the surface of a liquid. For this purpose, usually platinum rings are used, since they form zero contact angles with the outermost and innermost peripheries of the wire. Determine the force required to pull such a ring of diameter 0.4 m from water above the
The surface tension of molten glass is of prime importance in practically all operations of manufacturing glass wares, beginning with the fining process, and continuing until the glass becomes rigid.
Full text of “Measurement of Surface Tension” JAEGER’S METHOD When the column of liquid that rise& in a tube dipping vertically into a liquid is slowly forced down by applied gas pressure, it is observed that the pressure steadily increases to a well-marked maxi- mum just before a bubble escapes from the end of the tube. The value of this maximum depends upon the surface tension and the
The surface tension of a liquid mixture is not a simple function of the surface tensions of the pure liquids. Also, the composition of the bulk phase and the composition at the vapor-liquid
Surface Tension as a Function of Temperature and Concentration of Liquids. U. P. SHINDE1, S. S. CHOUGULE1, follows 1) Capillary rise method 2) Jaeger’s method 3) Ferguson and Kennedy’s method 4) Quincke’s method etc. From the above methods the capillary rise method is simple, easy and less expensive.
Young’s equation is the basic relationship for calculating the solid surface tension gs from the contact angle q and the surface tension of the liquid gl As one can see, the equation contains the two parameters q and gl which can readily be measured, and two other parameters gs …
Methods SINTERFACE
Liquid-Liquid Interfacial Tension Measurements
surface tension of the liquid. This phenomenon is used to determine the surface tension using stalagmometric method (method of counting drops). Stalagmometer (Fig. 1a) is a device made up of a glass bulb with marked above and below the bulb indicators designating specific volume of liquid, ended capillary. To measure the surface tension of the test liquid fill of stalagmometer and allow it to
Show transcribed image text A method for determining the surface tension of a liquid is to find the force needed to pull a wire ring from the surface of a liquid. For this purpose, usually platinum rings are used, since they form zero contact angles with the outermost and innermost peripheries of the wire. Determine the force required to pull such a ring of diameter 0.4 m from water above the
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
The temperature coefficient of the surface tension (dσ/dT) of liquid stainless steel was found to change from negative to positive at a sulphur concentration of about 30 mass ppm in the steel. Nitrogen was found to have little effect on the surface tension of liquid stainless steel.
The procedure to determine the surface tension of a given liquid is as follows: Measure the ambient temperature and fill the burette with the liquid to be analyzed. The drop formation time is regulated slowly enough to avoid hydrodynamics effects.

One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube. By setting the two forces above equal, we find the surface tension to be: By setting the two forces above equal, we find the surface tension to be:
The Surface tensions of molten glass part I surface
Measurement of dynamic surface tension by the oscillating
Determination of the surface tension of liquid stainless
An optical measuring method has been applied to determine the dynamic surface tension of aqueous solutions of heptanol. The method uses the frequency of an oscillating liquid droplet as an indicator of the surface tension of the liquid. Droplets with diameters in the range between 100 and 200 μm
On the measurement of the surface tension of a small
Critical Assessment of the Surface Tension determined by
Full text of “Measurement of Surface Tension” JAEGER’S METHOD When the column of liquid that rise& in a tube dipping vertically into a liquid is slowly forced down by applied gas pressure, it is observed that the pressure steadily increases to a well-marked maxi- mum just before a bubble escapes from the end of the tube. The value of this maximum depends upon the surface tension and the
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
This Test Guideline describes methods to determine the surface tension (in N/m) of aqueous solutions. The methods are based on the measurement of the force which it is necessary to exert vertically on a stirrup or ring, in contact with the surface of the liquid, in order to separate it from the surface, or on a plate, with an edge in contact
Methods SINTERFACE
Surface Tension Prediction for Liquid Mixtures
The Surface tensions of molten glass part I surface
Surface Tension as a Function of Temperature and Concentration of Liquids. U. P. SHINDE1, S. S. CHOUGULE1, follows 1) Capillary rise method 2) Jaeger’s method 3) Ferguson and Kennedy’s method 4) Quincke’s method etc. From the above methods the capillary rise method is simple, easy and less expensive.
Methods SINTERFACE
Surface Tension Prediction for Liquid Mixtures
On the measurement of the surface tension of a small
8 Knowing the temperature in laboratory, determine the water surface tension using values from the Table 1, and calculate the surface tensions of studied liquids according to the equation [7]. Experimental procedure (drop-counting method):
Conducting Surface Tension Measurements For Compliance
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
On the measurement of the surface tension of a small
Surface tension may be regarded as the resistance offered by liquid water to forces attempting to deform or break through the surface film of water. It is an interesting property and, for water, the surface tension measured in Newton’s per meter (N m −1 ), is high and shows a slight increase as the temperature falls from 100 (0.0589 N m −1 ) to 0 °C (0.0765 N m −1 ).
SURFACE PROPERTIES OF FUSED SALTS AND GLASSES I SESSILE
Surface Tension Prediction for Liquid Mixtures
Solved A Method For Determining The Surface Tension Of A
approach one can calculate the surface tension of water/surfactant solution as a function of surfactant surface concentration, Γ. This method predicts the correct qualitative dependence σ(Г) and reproduces other important trends. However, the direct application of this approach to calculation of σcmc would be challenging as it would involve the calculation or measurement of the surface
Conducting Surface Tension Measurements For Compliance
The expression which relates the liquid surface tension with the pressure difference is obtained by performing an analysis of the work done by the air pressure to increase the volume and the surface area of the bubble while it expands into the sample liquid Equation 1.
OECD iLibrary Test No. 115 Surface Tension of Aqueous
Instruction for the Laboratory of Biophysics